Finger-Prick Blood Test Offers Accessible Alzheimer's Detection Breakthrough | Quick Digest
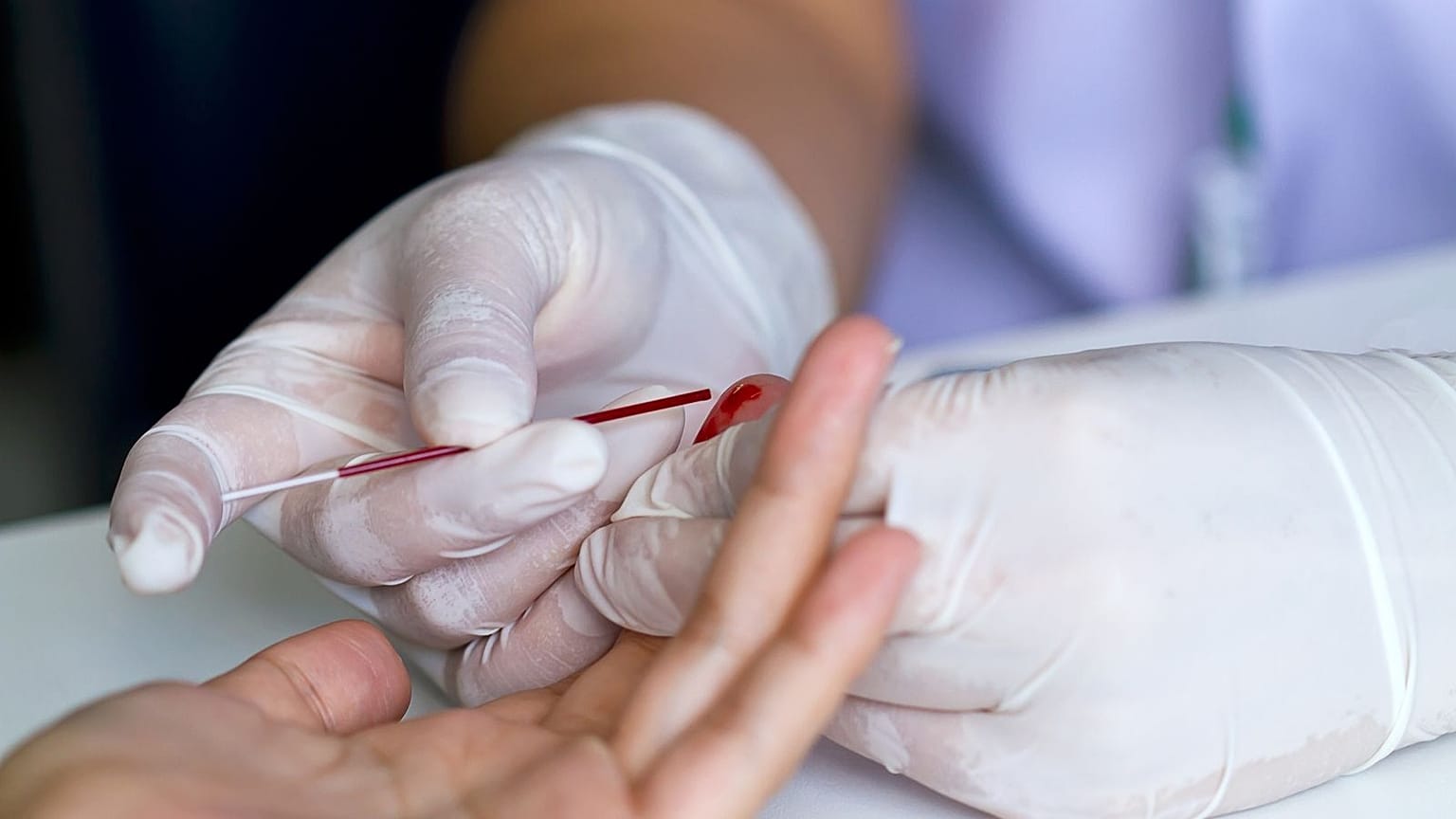
An international study published in Nature Medicine validated a finger-prick blood test for Alzheimer's biomarkers. This method allows easy at-home sample collection and analysis, overcoming logistical challenges for widespread research and eventual early detection, though it's not yet clinically available.
International study validates finger-prick blood test for Alzheimer's biomarkers.
Test accurately detects p-tau217, GFAP, and NfL from dried blood spots.
Enables convenient at-home sample collection and mailing without refrigeration.
Research published in Nature Medicine, led by Banner Health and University of Exeter.
Primarily for research and screening; requires further validation for clinical use.
Holds global potential, especially for regions with limited healthcare infrastructure.
A groundbreaking international study has revealed that Alzheimer's disease biomarkers can be accurately detected using simple finger-prick blood samples, a development reported by researchers and corroborated across numerous credible sources, including NIHR, Alzforum, Sky News, and EurekAlert!. Published in the prestigious journal *Nature Medicine* around January 5-6, 2026, the research was led by the US-based Banner Health and the University of Exeter Medical School in the UK, with contributions from seven European medical centers, including the University of Gothenburg in Sweden. This innovative method involves collecting a few drops of blood from a fingertip, which are then dried on a card and can be mailed to laboratories without requiring refrigeration or prior processing.
The study, which involved 337 participants, demonstrated that levels of key Alzheimer's biomarkers, such as p-tau217, Glial Fibrillary Acidic Protein (GFAP), and neurofilament light (NfL), in these finger-prick samples closely matched results from standard blood tests and accurately identified Alzheimer's disease-related changes in spinal fluid with an 86% accuracy for p-tau217. This breakthrough is significant because current diagnostic methods for Alzheimer's, such as brain scans or spinal fluid tests, are often invasive, expensive, and limited in accessibility, particularly in remote or underserved areas globally. While traditional venous blood tests are simpler, they still require trained personnel and specific storage conditions.
Researchers emphasize that while this finger-prick technique holds immense promise for transforming Alzheimer's research, enabling large-scale screening studies and including diverse, historically underrepresented populations, it is "still years away from clinical use" and requires "significant additional research and validation" before becoming a routine diagnostic tool. For now, its primary application lies in advancing research and streamlining the recruitment process for clinical trials. The ability to self-collect samples at home could fundamentally change how global brain disease research is conducted, making it more accessible worldwide. This development offers a hopeful pathway toward earlier and more equitable detection strategies for Alzheimer's disease.
Read the full story on Quick Digest